Kc 730℃ 2.18×10^6 H2(g) + Br2(g) ⇌ 2HBr(g) 12.0 L HBr 3.20 mol
The equilibrium constant Kc for the reaction
H2(g) + Br2(g) ⇌ 2HBr(g)
is 2.18×10^6 at 730°C.
Starting with 3.20 moles of HBr in a 12.0 L reaction vessel,
calculate the concentrations of H2, Br2, and HBr at equilibrium.
반응 전 HBr의 몰농도를 계산하면,
[HBr] = 3.20 mol / 12.0 L = 0.267 M
ICE 도표를 작성하면,
............. H2(g) + Br2(g) ⇌ 2HBr(g)
초기(M) . 0 ......... 0 ........... 0.267
변화(M) . +x ....... +x .......... –2x
평형(M) . x ......... x ............ 0.267–2x
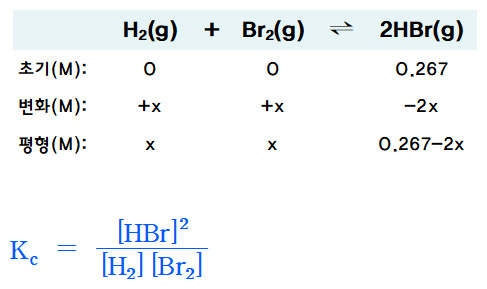
[그림] ICE 도표 및 평형 상수 식.
( 참고 https://ywpop.tistory.com/7136 )
Kc = [HBr]^2 / [H2] [Br2]
2.18×10^6 = (0.267–2x)^2 / (x) (x)
2.18×10^6 = (0.267–2x)^2 / (x)^2
2.18×10^6 = [(0.267–2x) / (x)]^2
(2.18×10^6)^(1/2) = (0.267–2x) / (x)
1476.48x = 0.267–2x
1478.48x = 0.267
x = 0.267 / 1478.48
= 0.00018059 M
≒ 0.000181 M
답:
[H2] = [Br2] = x = 1.81×10^(-4) M
[HBr] = 0.267 – 2(0.000181)
= 0.266638 ≒ 0.267 M
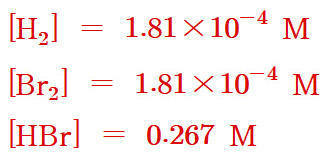
[ 관련 글 https://ywpop.blogspot.com/2024/06/1374-g-h2-7031-g-br2-200-l-700-k-0566-g.html ]
A mixture of 1.374 g of H2 and 70.31 g of Br2
is heated in a 2.00 L vessel at 700 K.
These substances react as follows:
H2(g) + Br2(g) ⇌ 2HBr(g)
At equilibrium the vessel is found to contain 0.566 g of H2.
a. Calculate the equilibrium concentrations of H2, Br2 and HBr.
b. Calculate Kc.

[키워드] H2(g) + Br2(g) ⇌ 2HBr(g) 평형 기준, H2 + Br2 ⇌ 2HBr 평형 기준
'일반화학 > [15장] 화학 평형' 카테고리의 다른 글
| 2BrCl(g) ⇌ Br2(g) + Cl2(g) 500 K 0.500 atm BrCl(g) 0.040 atm (0) | 2022.11.16 |
|---|---|
| 3.0 L 2.4 mol Cl2 1.0 mol NOCl 4.5×10^(-3) mol NO K (1) | 2022.11.13 |
| 이론적 평형상수. ([HA]/[A^-]) × ([B^-]/[HB])의 값 (1) | 2022.11.10 |
| H2(g) + I2(g) ⇌ 2HI(g) Kc 56 435℃ 0.045 mol H2 I2 10.0 L (0) | 2022.11.07 |
| 2NOBr(g) ⇌ 2NO(g) + Br2(g) 25℃ 34% 0.25 atm Kp Kc (0) | 2022.11.03 |
| 평형상수 Kc. [Br2] = 0.124 M, [Cl2] = 0.237 M, [BrCl] = 0.450 M (1) | 2022.11.02 |
| 르 샤틀리에. F2 + Cl2 ⇌ 2ClF + 열 반응에서 Cl2 양 (0) | 2022.11.02 |
| 2NOBr(g) ⇌ 2NO(g) + Br2(g) density 4.495 g/L 4.086 g/L (1) | 2022.10.22 |

댓글