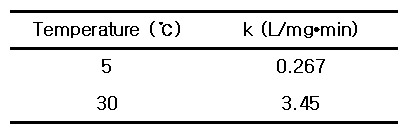
Mycobacterium avium chlorine dioxide ClO2 5℃ 0.267 30℃ 3.45
이산화 염소에 의해 미코박테리움 아비움이
비활성화되는 반응의 활성화 에너지(kJ)는 얼마인가?
Mycobacterium avium, a human pathogen responsible for respiratory infections, is sometimes found in hot tubs. M. avium can be inactivated by many disinfectants including chlorine, chlorine dioxide, and ozone.
For inactivation by chlorine dioxide, the following rate constants were obtained.
What is the activation energy (in kJ) for the inactivation of M. avium by chlorine dioxide?
---------------------------------------------------
> k_1 = 0.267, T_1 = 273 + 5 = 278 K
> k_2 = 3.45, T_2 = 273 + 30 = 303 K
아레니우스 식. 속도 상수의 온도 의존성
ln(k_2 / k_1) = Ea/R (1/T_1 – 1/T_2)
( R = 8.314 J/K•mol )
( 참고 https://ywpop.tistory.com/7288 )
Ea = [ln((k_2) / (k_1)) × 8.314] / [(1/T_1) – (1/T_2)]
= [ln((3.45) / (0.267)) × 8.314] / [(1/278) – (1/303)]
= 71682 J/mol
답: 71.7 kJ/mol
[키워드] Mycobacterium avium 기준문서, Mycobacterium avium dic
'일반화학 > [14장] 화학반응 속도론' 카테고리의 다른 글
| sucrose water first order 6.17×10^(-4) s^-1 t_1/2 minute 3/4 (0) | 2022.11.09 |
|---|---|
| 속도 상수의 단위. rate constant unit (0) | 2022.11.05 |
| NO H2 2.0×10^(-6) mol L-1 rate 7.86×10^(-3) mol L-1 s-1 (0) | 2022.11.05 |
| SO2Cl2 first order 600 K 2.3×10^5 s 320℃ 2.2×10^(-5) s-1 (0) | 2022.11.04 |
| 단백질 이합체화 2차 속도 상수 6.2×10^(-3) /M•s (1) | 2022.10.29 |
| 반감기가 3일인 방사성 폐수의 농도가 10 mg/L (0) | 2022.10.28 |
| 속도 상수 2배 반응 활성화 에너지. 295 K와 305 K (1) | 2022.10.27 |
| 물고기 근육 속의 박테리아 가수 분해 속도 (0) | 2022.10.27 |
댓글