C6H5COOH(aq) + CH3COO^-(aq) ⇌ C6H5COO^-(aq) + CH3COOH(aq) is 3.6
The equilibrium constant for the reaction
C6H5COOH(aq) + CH3COO^-(aq) ⇌ C6H5COO^-(aq) + CH3COOH(aq)
is 3.6 at 25℃.
If Ka for CH3COOH is 1.8×10^(-5),
what is the acid dissociation constant for C6H5COOH?
---------------------------------------------------
▶ 참고: 이론적 평형상수
[ https://ywpop.tistory.com/4154 ]
---------------------------------------------------
C6H5COOH(aq) + CH3COO^-(aq) ⇌ C6H5COO^-(aq) + CH3COOH(aq)
K = [C6H5COO^-] [CH3COOH] / [C6H5COOH] [CH3COO^-]
CH3COOH(aq) ⇌ H^+(aq) + CH3COO^-(aq)
Ka1 = [H^+] [CH3COO^-] / [CH3COOH]
C6H5COOH(aq) ⇌ H^+(aq) + C6H5COO^-(aq)
Ka2 = [H^+] [C6H5COO^-] / [C6H5COOH]
H^+(aq) + CH3COO^-(aq) ⇌ CH3COOH(aq)
Ka3 = [CH3COOH] / [H^+] [CH3COO^-]
아세트산 이온화의 역반응이므로,
Ka3 = 1 / Ka1
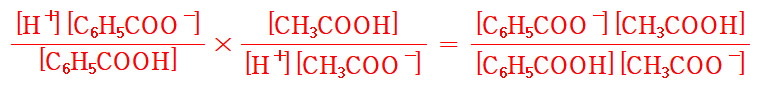
Ka2 × Ka3 = K 이므로,
Ka2 = K / Ka3
= 3.6 / (1 / (1.8×10^(-5)))
= 6.5×10^(-5)
답: 6.5×10^(-5)
'일반화학 > [15장] 화학 평형' 카테고리의 다른 글
| 평형상수식. 2NH3(g) + CO2(g) ⇌ N2CH4O(s) + H2O(g) (0) | 2022.01.03 |
|---|---|
| Kc 계산. 2SO2(g) + O2(g) ⇌ 2SO3(g) (0) | 2021.12.16 |
| COBr2(g) ⇌ CO(g) + Br2(g) 73℃ Kc 0.190 2.00 L COBr2 0.0500 mol (0) | 2021.12.12 |
| 이론적 평형상수. H(g) + Br(g) ⇌ HBr(g) (0) | 2021.12.11 |
| 25℃ Kc = 7.0×10^25 2SO2(g) + O2(g) ⇌ 2SO3(g) (0) | 2021.12.02 |
| Kc 계산. H2O(g) + CO(g) ⇌ CO2(g) + H2(g) (0) | 2021.12.02 |
| 이론적 평형상수. H2N-CH2-COOH ⇌ +H3N-CH2-COO^- (0) | 2021.11.25 |
| 420℃ H2(g) + CO2(g) ⇌ H2O(g) + CO(g) K = 0.10 (0) | 2021.11.18 |


댓글