pH 10에서 EDTA의 구조
What is the structure of EDTA at pH 10?
---------------------------------------------------
At pH 10, ethylenediaminetetraacetic acid (EDTA) exists in its fully deprotonated form, meaning that all of its acidic protons are dissociated.
The structure of EDTA at pH 10 is a highly charged anion with a complex of four carboxylate groups and two nitrogen atoms that can bind to metal ions.
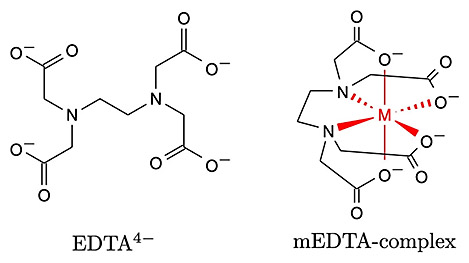
[그림] fully deprotonated form = EDTA^4- = Y^4-.
[출처] From the Free Ligand to the Transition Metal Complex: FeEDTA^- Formation Seen at Ligand K-Edges
Sebastian Eckert*, Eric J. Mascarenhas, Rolf Mitzner, Raphael M. Jay, Annette Pietzsch, Mattis Fondell, Vinícius Vaz da Cruz, and Alexander Föhlisch
[ 관련 글 https://ywpop.tistory.com/4811 ] 몇 가지 일반적인 리간드
[키워드] EDTA^4- 기준문서, EDTA 기준문서
'일반화학 > [23장] 전이 금속과 배위 화학' 카테고리의 다른 글
| 옥살레이트-철 착화합물. K3[Fe(C2O4)3]*3H2O (4) | 2024.11.04 |
|---|---|
| CuCl과 CuCl2 (0) | 2024.10.31 |
| 지르코늄의 산화수. Zr의 산화수 (1) | 2024.03.29 |
| [명명법] [Co(NH3)4CO3]NO3 [Co(NH3)5Cl]Cl2 (4) | 2023.04.18 |
| [Co(NH3)4Cl2]^+ 기하 이성질체 개수와 Co의 산화수 (3) | 2023.02.08 |
| [Mn(CN)6]^5-, [Mn(CN)6]^4-, [Mn(CN)6]^3- 전자배치 (1) | 2022.12.13 |
| [Mn(CN)6]^4- + e^- ⇌ [Mn(CN)6]^5- reaction (0) | 2022.12.13 |
| [Co(en)2(H2O)Br]^2+ 착이온의 배위수, 산화수 (0) | 2022.12.01 |

댓글