[H2O(g)] = 7.9×10^(-2) M, [CO2(g)] = 0.93 M, [O2(g)] = 2.4×10^(-3) M
The formation of glucose from water and carbon dioxide
is an extremely important reaction for life to exist.
Plants perform this reaction through the process of photosynthesis,
creating the base of the food chain. The unbalanced reaction is
H2O(g) + CO2(g) ⇌ C6H12O6(s) + O2(g)
At a particular temperature,
the following equilibrium concentrations were found:
[H2O(g)] = 7.9×10^(-2) M, [CO2(g)] = 0.93 M, [O2(g)] = 2.4×10^(-3) M.
Calculate the value of K at this temperature for the reaction
that produces 1 mol of glucose.
---------------------------------------------------
균형 맞춘 반응식
6H2O(g) + 6CO2(g) ⇌ C6H12O6(s) + 6O2(g)
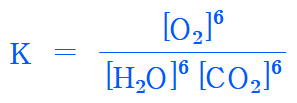
K = [O2]^6 / [H2O]^6 [CO2]^6
= ( [O2] / [H2O] [CO2] )^6
= ( (2.4×10^(-3)) / ((7.9×10^(-2)) (0.93)) )^6
= 1.2151×10^(-9)
답: 1.2×10^(-9)
[키워드] 6CO2(g) + 6H2O(g) ⇌ C6H12O6(s) + 6O2(g), 6CO2 + 6H2O ⇌ C6H12O6 + 6O2, CO2(g) + H2O(g) ⇌ C6H12O6(s) + O2(g), CO2 + H2O ⇌ C6H12O6 + O2
'일반화학 > [15장] 화학 평형' 카테고리의 다른 글
| 백금 촉매 자동차 촉매 변환기 일산화탄소 산화 촉진 (2) | 2022.07.13 |
|---|---|
| A(g) ⇌ C(g) + D(g) 1.0 mol A 0.4 L 0.2 mol C (0) | 2022.07.05 |
| 이론적 평형상수. 2NH3(g) + 3I2(g) ⇌ 6HI(g) + N2(g) (0) | 2022.06.20 |
| H2(g) + I2(g) ⇌ 2HI(g) Kc 57 at 700 K 1 mol H2 1 mol I2 in 1 L (0) | 2022.06.20 |
| 350 K 10 L 2 mol SO3 SO2 : SO3 0.663 (0) | 2022.05.18 |
| 0.050 mol CH2O 500 mL CH2O(g) = H2(g) + CO(g) 0.066 mol/L (1) | 2022.05.13 |
| 2BrCl ⇌ Br2 + Cl2 Kc = 0.145 0.220 mol BrCl 250 mL CCl4 (0) | 2022.05.13 |
| CO2 H2 3.0 mol 500℃ CO 1.0 mol H2O 1.0 mol (0) | 2022.04.09 |


댓글