산-염기 반응. CH3COOH + NH3. CH3COOH + CH3NH2
▶ 산-염기 반응: CH3COOH(aq) + NH3(aq)
( acetic acid and ammonia reaction in water )
> CH3COOH(aq) + NH3(aq) → CH3COONH4(aq)
( CH3COOH + NH3 → CH3COONH4 )
> NH3 + H2O ⇌ NH4OH
> NH4OH + CH3COOH → CH3COONH4 + H2O
( CH3COO^- • NH4^+ )
( 참고 https://www.chemguide.co.uk/organicprops/anhydrides/nitrogen.html )
> 1단계: NH3 + H2O ⇌ NH4OH
> 2단계: NH4OH + CH3COOH → CH3COONH4 + H2O
> 전체: NH3 + CH3COOH → CH3COONH4
---> CH3COOH: 양성자 주게 산 (브뢴스테드 산)
---> NH3: 양성자 받게 염기 (브뢴스테드 염기)

[그림] H^+ + NH3 → NH4^+
> H^+: 전자쌍 받게 산 (루이스 산)
> NH3: 전자쌍 주게 염기 (루이스 염기)
( 참고: 산-염기의 정의 https://ywpop.tistory.com/2696 )
▶ 산-염기 반응: CH3COOH(aq) + CH3NH2(aq)
( acetic acid and methylamine reaction in water )
> CH3COOH(aq) + CH3NH2(aq) → CH3COONH3CH3(aq)
( CH3COOH + CH3NH2 → CH3COONH3CH3 )
> CH3NH2 + H2O ⇌ CH3NH3OH
> CH3NH3OH + CH3COOH → CH3COONH3CH3 + H2O
( CH3COO^- • CH3NH3^+ )
> 1단계: CH3NH2 + H2O ⇌ CH3NH3OH
> 2단계: CH3NH3OH + CH3COOH → CH3COONH3CH3 + H2O
> 전체: CH3NH2 + CH3COOH → CH3COONH3CH3
---> CH3COOH: 양성자 주게 산 (브뢴스테드 산)
---> CH3NH2: 양성자 받게 염기 (브뢴스테드 염기)

[그림] H^+ + CH3NH2 → CH3NH3^+
> H^+: 전자쌍 받게 산 (루이스 산)
> CH3NH2: 전자쌍 주게 염기 (루이스 염기)
The salt is called methylammonium ethanoate. It is just like ammonium ethanoate, except that one of the hydrogens has been replaced by a methyl group.

---------------------------------------------------
[참고] 아래 반응식은 어떻게 된 것인가?
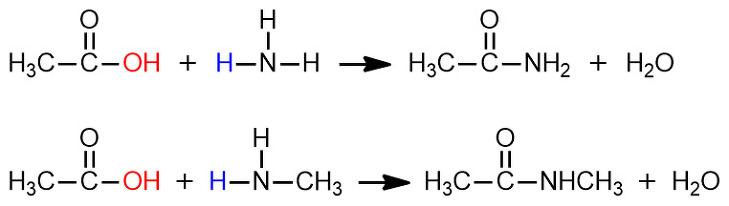
> 진한 황산(H2SO4)과 같은 적절한 촉매 존재 하에서,
acetic acid와 ammonia를 반응시키면, ammonium acetate가 생성됩니다.
> ammonium acetate를 고온(~200℃)으로 가열시키면, 물 분자가 빠지면서 acetamide가 생성됩니다.
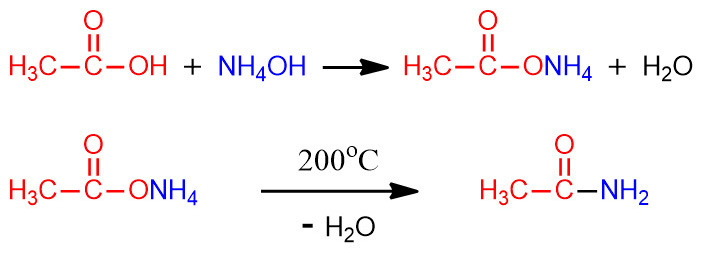
> 1단계: NH3 + H2O ⇌ NH4OH
> 2단계: CH3COOH + NH4OH → CH3COONH4 + H2O
> 3단계: CH3COONH4 → CH3CONH2 + H2O
> 전체: CH3COOH + NH3 → CH3CONH2 + H2O
진한 황산(H2SO4)과 같은 적절한 촉매 존재 하에서, 아세트산(CH3COOH)과 암모니아(NH3)를 반응시키면 아미드 화합물이 생성됩니다. 이 과정을 “아미드화”라고 합니다.
When acetic acid (CH3COOH) reacts with ammonia (NH3) in the presence of a suitable catalyst, such as concentrated sulfuric acid (H2SO4), the reaction leads to the formation of an amide compound. This process is known as amidation.
Amidation is the process by which the –CONH2 structure is created.
[ 관련 글 https://ywpop.tistory.com/12570 ]
산-염기 반응. CH3CH2COOH + NH3. RCOOH + NH3
[키워드] CH3COOH + NH3 반응 기준, CH3COOH + CH3NH2 반응 기준, 약산-약염기 반응 기준, RCOOH + NH3 반응 기준, 브뢴스테드 산-염기 기준, 루이스 산-염기 기준, NH3는 양성자 받게 염기 기준, NH3는 브뢴스테드 염기 기준, NH3는 전자쌍 주게 염기 기준, NH3는 루이스 염기 기준, 브뢴스테드 산-염기 사전, 루이스 산-염기 사전, NH3는 양성자 받게 염기 사전, NH3는 브뢴스테드 염기 사전, NH3는 전자쌍 주게 염기 사전, NH3는 루이스 염기 사전, H^+ + NH3 → NH4^+ 기준, H^+(aq) + NH3(aq) → NH4^+(aq) 기준, H^+ + NH3 → NH4^+ dic, H^+(aq) + NH3(aq) → NH4^+(aq) dic
'일반화학 > [16장] 산-염기 평형' 카테고리의 다른 글
| 0.1 M CH3COOH 용액의 pH (0) | 2018.12.09 |
|---|---|
| NH3 ← HCl 당량점의 pH (0) | 2018.12.04 |
| 반-당량점에서 pH = pKa ★ (1) | 2018.12.01 |
| 산성염 염기성염 중성염 분류 (3) | 2018.11.27 |
| 0.05 M HCN 용액의 pH + 모든 화학종의 농도 ★ (0) | 2018.10.30 |
| 0.001 M H2CO3 용액의 pH (2) | 2018.10.24 |
| 짝산-짝염기 쌍. HCl + H2O. NaOH + H2O (0) | 2018.10.08 |
| HCl + CaO 산-염기 반응. CaO는 염기성 물질 (0) | 2018.09.24 |
댓글