methyl isocyanate (CH3NC) isomerizes to acetonitrile (CH3CN)
기체 상태에서 메틸 이소시아네이트(CH3NC)는
아세토나이트릴(CH3CN)으로 이성화한다.
25℃에서 ΔH° = –89.5 kJ/mol, ΔG° = –73.8 kJ/mol이다.
100℃에서 이 반응의 평형 상수를 찾아라.
In the gas phase, methyl isocyanate (CH3NC) isomerizes to acetonitrile (CH3CN),
H3C–N≡C(g) ⇌ H3C–C≡N(g)
with ΔH° = –89.5 kJ/mol and ΔG° = –73.8 kJ/mol at 25℃.
Find the equilibrium constant for this reaction at 100°C.
What is the equilibrium constant, Kp, for this reaction at 100℃,
assuming that the enthalpy and entropy of reaction
are independent of temperature.
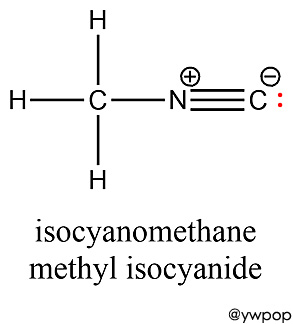
[참고] CH3NC는
methyl isocyanate가 아니라, methyl isocyanide이다.
From ΔG = ΔH – TΔS
( 참고 https://ywpop.tistory.com/7438 )
ΔS = (ΔH – ΔG) / T
= ((–89.5) – (–73.8)) / (273+25)
= –0.0527 kJ/mol•K
---> 25℃ ΔS 값
ΔG = ΔH – TΔS
= (–89.5) – (273+100) (–0.0527)
= –69.8 kJ/mol
---> 100℃ ΔG 값
From ΔG = –RT lnK
( 참고 https://ywpop.tistory.com/10336 )
lnK = –ΔG / RT
K = e^[–ΔG / RT]
= e^[–(–69800) / ((8.314) (273+100))]
= 5.96×10^9
---> 100℃ 평형상수
답: 5.96×10^9
[ 관련 글 https://ywpop.blogspot.com/2024/04/methyl-isocyanate-methyl-isocyanide.html ]
methyl isocyanate와 methyl isocyanide를 혼동하지 말자
[키워드] CH3NC CH3CN ΔH° = -89.5 kJ/mol ΔG° = -73.8 kJ/mol 25℃ K 100℃, gas CH3NC CH3CN 25℃ ΔH° = -89.5 kJ/mol ΔG° = -73.8 kJ/mol 100℃ K, CH3NC 루이스 기준, CH3-NC 루이스 기준, CH3NCO 루이스 기준, CH3-NCO 루이스 기준
'일반화학 > [19장] 화학 열역학' 카테고리의 다른 글
| ΔG 계산. Cu|Cu2^+(0.0200 M)||Ag^+(0.0200 M)|Ag (0) | 2022.04.22 |
|---|---|
| ΔG 계산. C(s, diamond) → C(s, graphite) (0) | 2022.04.09 |
| standard entropy change CaF2(s) → CaF2(aq) at 298 K (0) | 2022.02.06 |
| 293 K에서 0.2699 V, 303 K에서 0.2669 V (0) | 2022.01.03 |
| Boudouard 2CO(g) ⇌ C(s) + CO2(g) 5.07 MPa 1.00 mol% CO (0) | 2021.12.11 |
| Boudouard 2CO(g) ⇌ C(s) + CO2(g) 101.3 kPa 1.00 mol% CO (0) | 2021.12.11 |
| 25℃ Kw 1.0×10^(-14) ΔG° H2O(l) ⇌ H^+(aq) + OH^-(aq) (0) | 2021.12.10 |
| ΔG 계산. 2NH3(g) + CO2(g) → NH2CONH2(aq) + H2O(l) (1) | 2021.11.29 |
댓글