NH4HS total pressure 0.658 atm 25℃ free energy
NH4HS total pressure 0.658 atm 25℃ free energy
Ammonium hydrogen sulfide, a stink bomb ingredient,
decomposes to ammonia and hydrogen sulfide.
NH4HS(s) ⇌ NH3(g) + H2S(g)
Calculate the standard free energy of formation of NH4HS(s) at 25℃
if the total pressure resulting from NH4HS
placed in an evacuated container is 0.658 atm at 25℃.
---------------------------------------------------
ICE 도표를 작성하면,
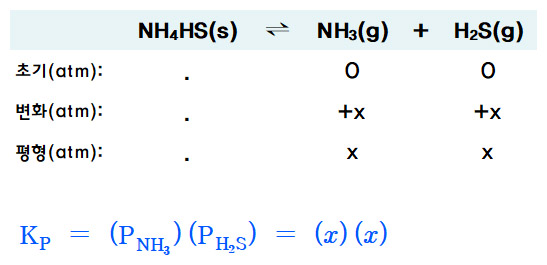
NH4HS(s) ⇌ NH3(g) + H2S(g)
평형에서, ...... x ............ x
Kp = (P_NH3) (P_H2S) = (x) (x)
x + x = 0.658 atm
x = 0.658 / 2 = 0.329 atm
Kp = (0.329)^2 = 0.108
ΔG° = –RT lnK
( 참고 https://ywpop.tistory.com/10336 )
= –(8.314) (273.15+25) ln(0.108)
= +5517 J/mol
= +5.52 kJ/mol
답: +5.52 kJ/mol
[ 관련 예제 https://ywpop.tistory.com/22793 ]
NH4HS(s) ⇌ NH3(g) + H2S(g) Kp = 0.108 at 25℃
[키워드] NH4HS(s) ⇌ NH3(g) + H2S(g) 기준문서